Indirect information on contact networks in chronic infections
Simon D.W. Frost


Acknowledgements
- Imperial
- Erik Volz
- Cambridge
- Lydia Drumright
- James Lester
- UNC Chapel Hill
- Sharon Weir
- Bar Ilan
- Yakir Berchenko
- Jonathan Rosenblatt
- Western
- Art Poon
- LSHTM
- Richard White
- UCLA
- Steve Shoptaw
- Pamina Gorbach
- Wisconsin-Madison
- Tony Goldberg
- Sam Sibley
- McGill
- Colin Chapman
- Oregon
- Nelson Ting
- Maria Jose Ruiz Lopez
- CDC
- Bill Switzer
Networks are important in disease transmission

Uganda Health Marketing Group/Johns Hopkins Center for Communication Programs,
Challenges in measuring networks
- Defining a contact
- Bounding networks in time, space, etc.
- Missing data
- Measuring weighted and dynamic networks
- Personal information and ethical concerns
- Exploiting indirect information about networks
Eames, Bansal, Frost, Riley. Epidemics (2014)
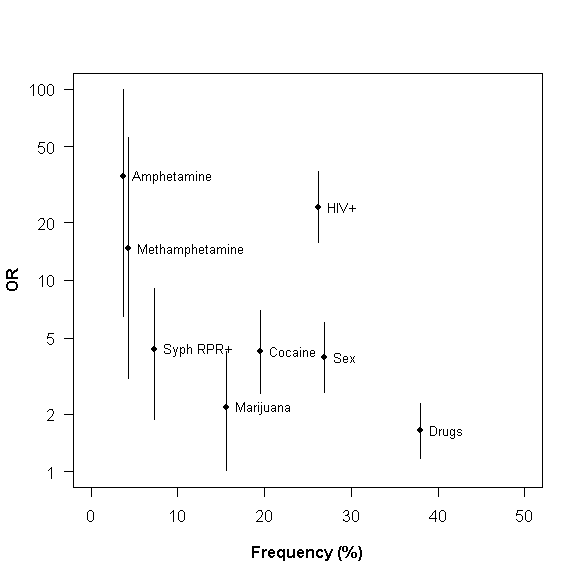
Unlinked infections in HIV
- 9/15 (60%) and 18/26 (69%): contact tracing of two networks in Cuba
- 7/36 (19.4%): a trial to test whether early initiation of antiretroviral therapy reduces transmission
- 40/148 (27.0%): a trial to determine the efficacy of genital herpes suppression in reducing HIV-1 transmission
- 20/149 (13.4%): a cohort of discordant couples in Lusaka, Zambia.
Resik et al. 2007, Eshleman et al. 2011, Campbell et al. 2011, Trask et al 2002
Why?
- Many reasons for either intentional or unintentional reporting of network data
- Long infectious period
- Low infection probability per contact
- Possibly many contacts
- Stigma
Proxies
- We can think of the sexual network as nested within the social network
- Egocentric network measures
- Venues
- Respondent-driven sampling
- The transmission network is nested within the contact network
- Pathogen sequencing
How many …?
- The degree of a node is a basic network measure
- Network scale-up method
- Asking how many individuals in a specific group someone knows
- Ask across multiple group types
- This approach can also be used to examine social networks by subpopulation
MeNS Network Study
- Priorities for Local AIDS Control Efforts (PLACE)
- Phase 1: identify key informants to identify venues
- Phase 2: interview staff of venues
- Phase 3: interview patrons of venues
- We used the PLACE protocol to study the role of bars, clubs, coffee shops, and other venues in structuring social, sexual, and drug-use networks in San Diego
w/Lydia Drumright, Sharon Weir
Network sizes
- We asked participants (n=609) to report network sizes
- 0, 1, 2-5, 6-10, 11+
- Groups (k=19) included:
- Names
- Ethnicity
- HIV
- Past sexually transmitted infections (STIs)
- Methamphetamine use
- Network sizes were assumed to follow a negative binomial distribution with group- and individual-level means
Zheng et al. (2006)
Network size by HIV
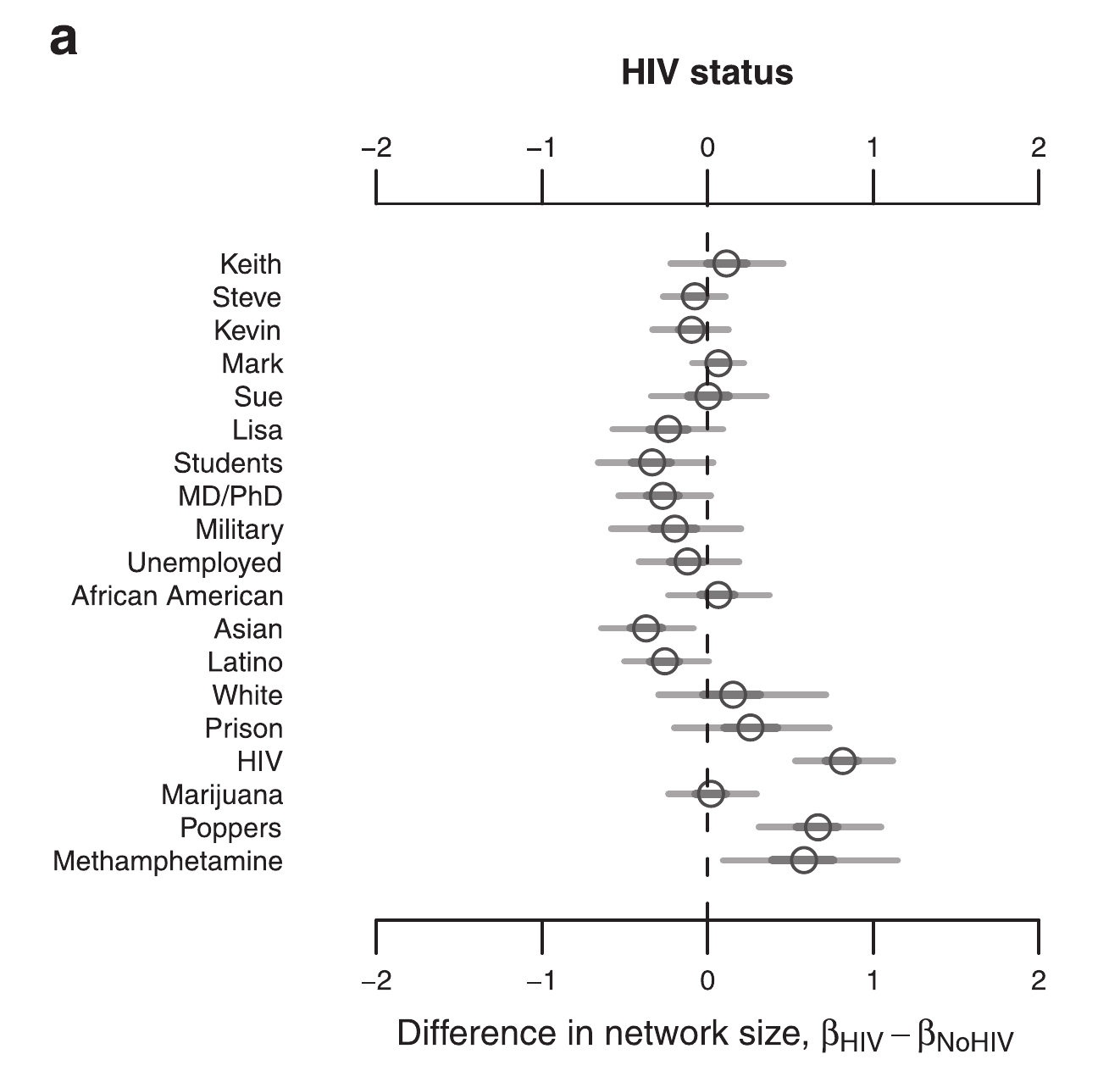
Drumright, Frost (2010)
Network size by HIV
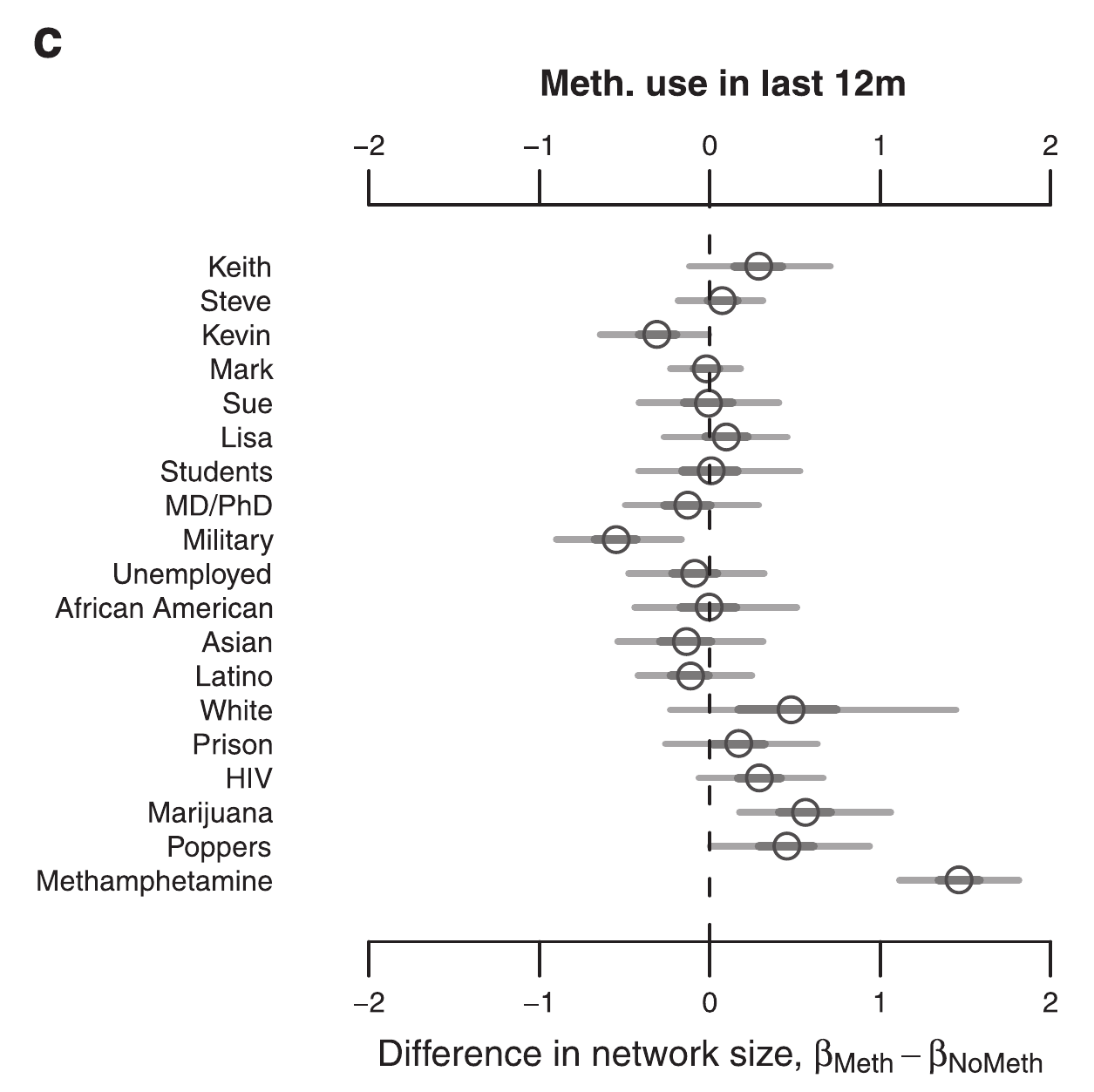
Drumright, Frost (2010)
Venues
- Time-location sampling is widely used in HIV surveillance
- Information on the venues is often ignored or treated as a nuisance parameter
- Information on specific venues may give insights into risk networks
- Risky people
- Risky places
Venues and networks
- Venues may also structure contact networks
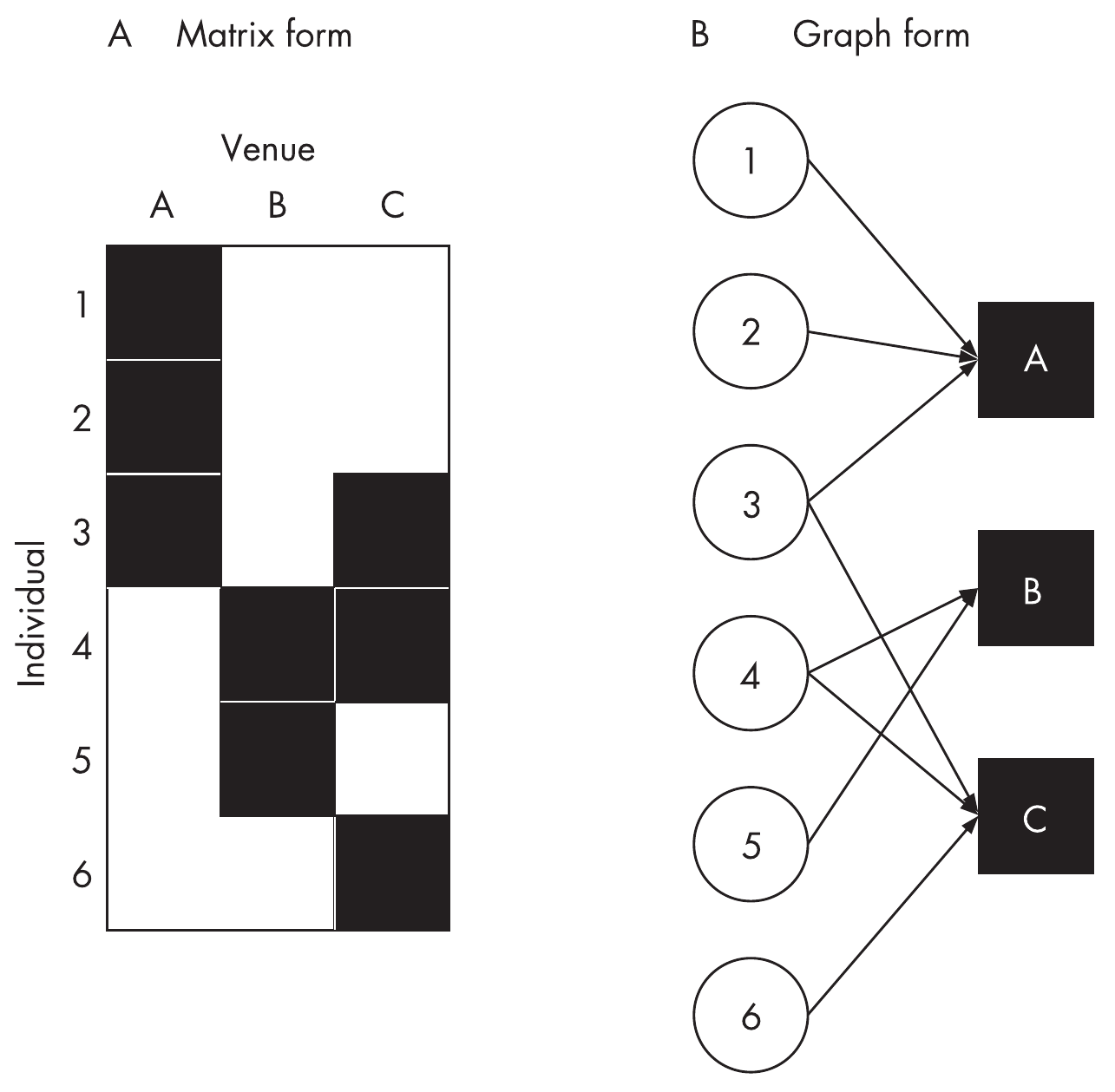
Frost (2007)
Venues in MeNS Network Study
- Venues stratify the population
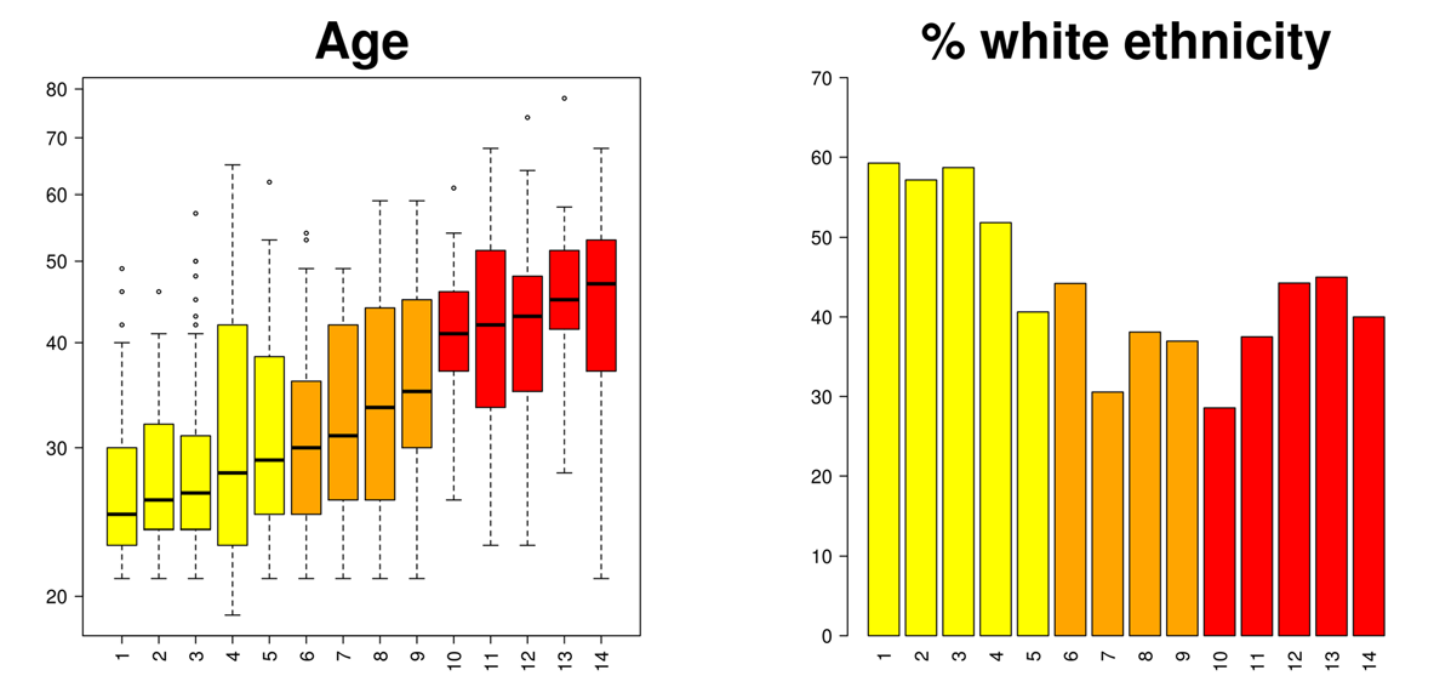
Drumright, Weir, Frost (2018)
Association with outcomes
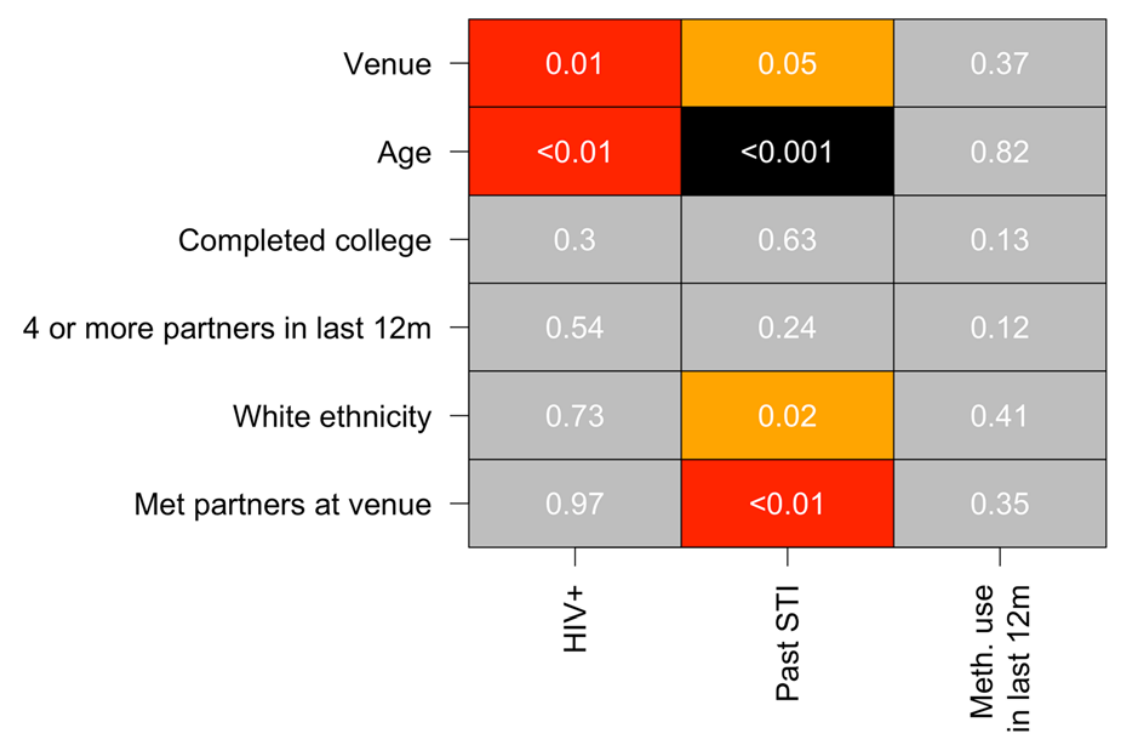
Drumright, Weir, Frost (2018)
Venues, HIV and STI
Drumright, Weir, Frost (2018)
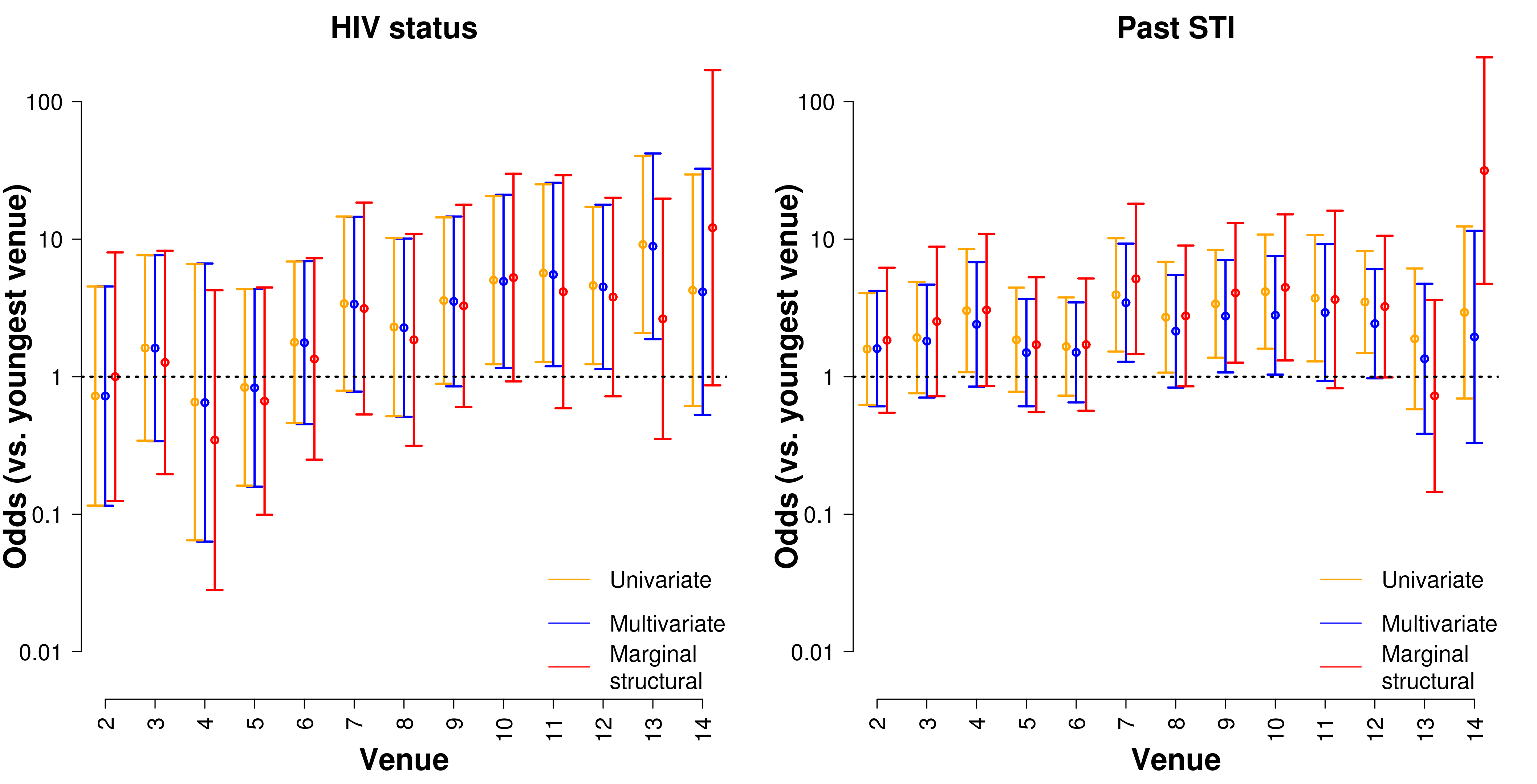
Network of venues
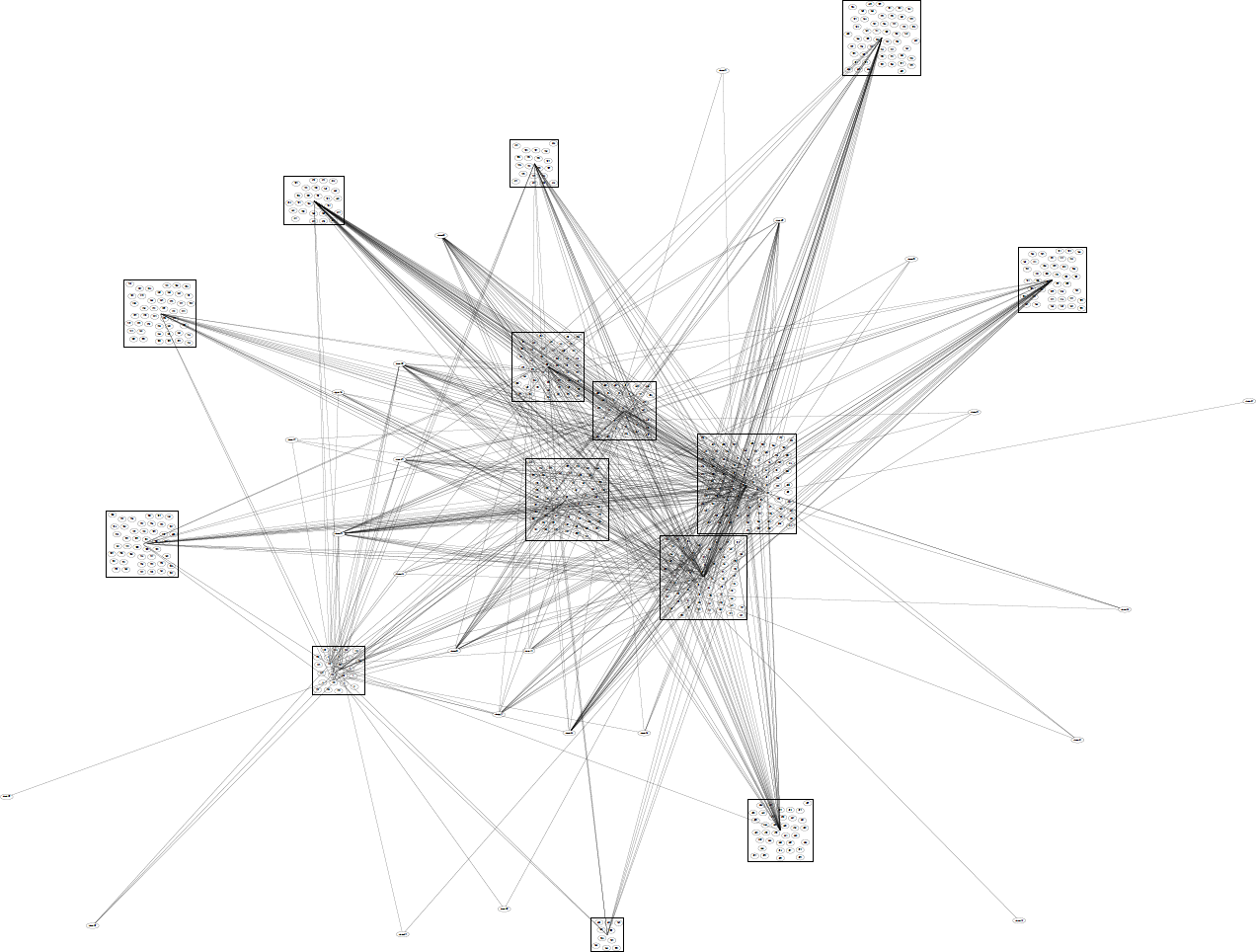
Alternative recruitment strategies
- Time-location sampling is common in HIV studies
- Relatively resource intensive
- Respondent-driven sampling is a popular method that harnesses peer referral networks
- Provides a rich, though hard to interpret, source of network data
Respondent-driven sampling
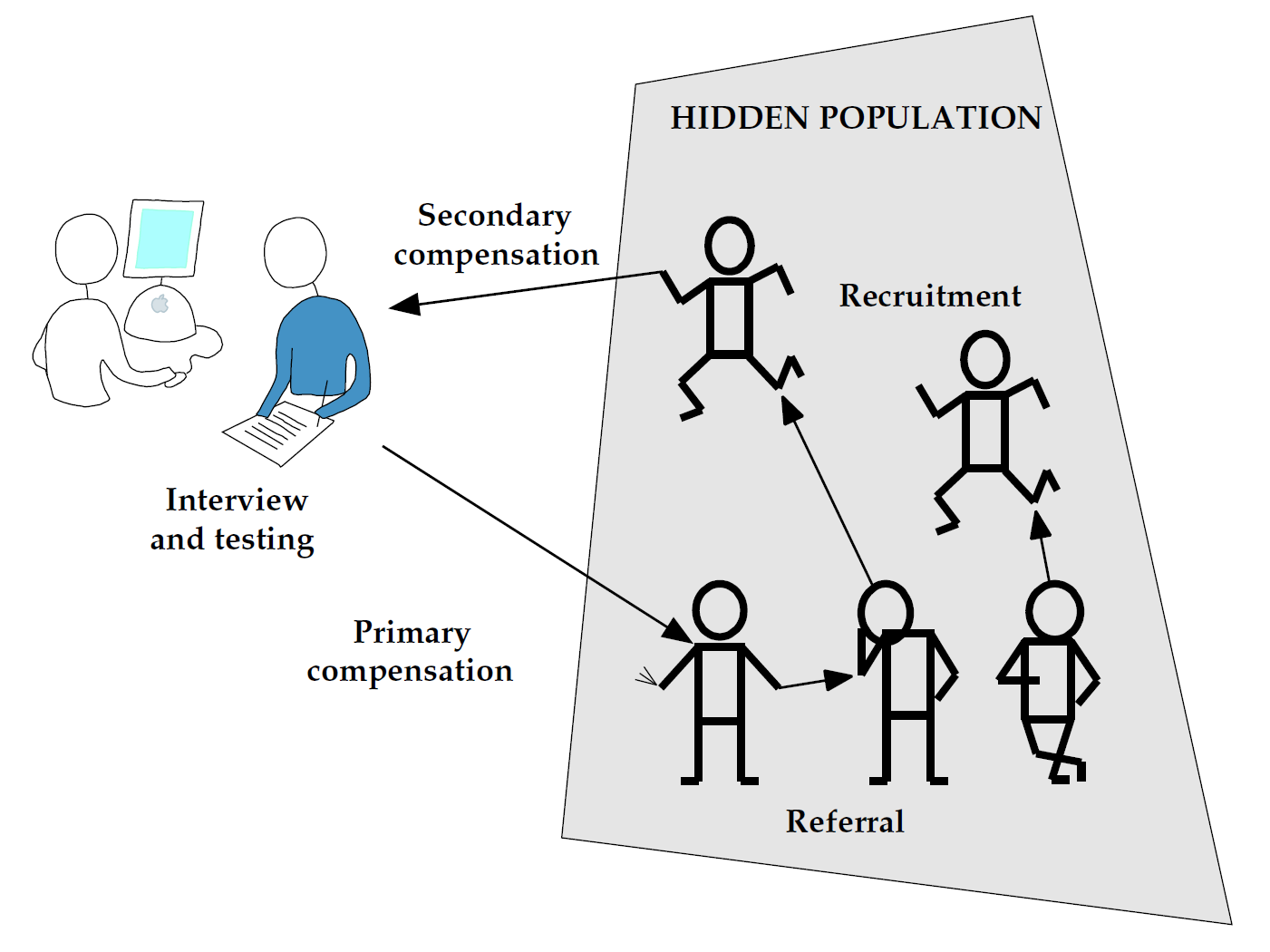
RDS produces trees
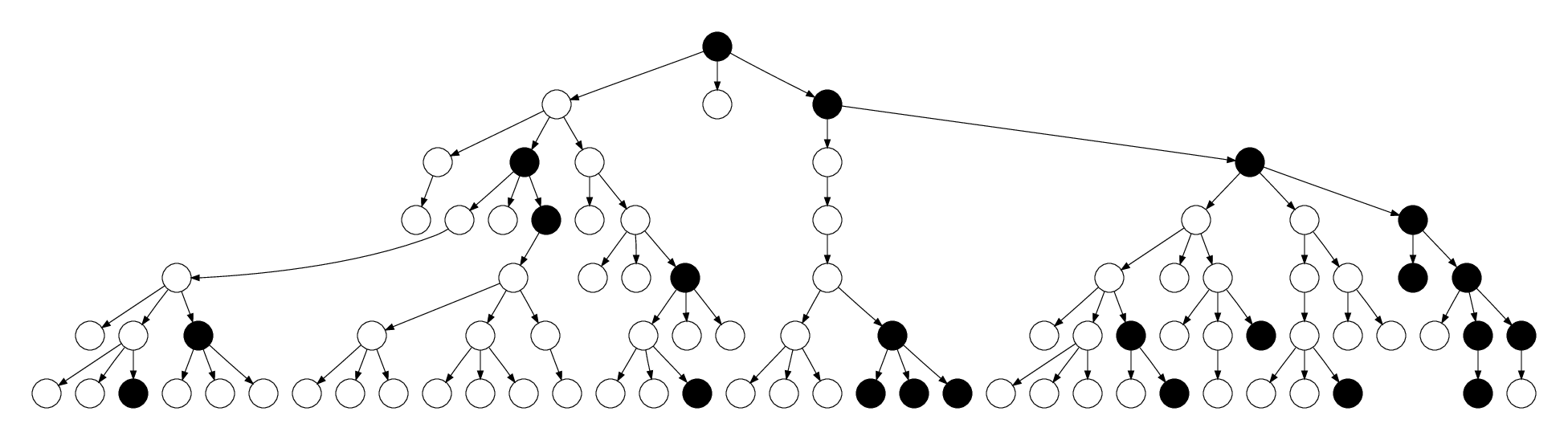
Recruitment proportional to degree
- RDS is often considered to be analogous to a random walk on a network
- Generates a random sample of edges rather than nodes
- Consequently, recruitment is proportional to degree
- Under this model, unbiased estimates e.g. of disease prevalence can be obtained by inversely weighting by the degree
RDS as an epidemic
- What are the dynamics of recruitment of individuals with different degrees during RDS?
- Compare the dynamics on a static network with those where there is simply heterogeneity in degree, but with no local network structure
- ‘Random mixing’ model of Volz, Eur Phys J B (2008)
Dynamics with Poisson-distributed degree, mean 5
Predictions of RDS
- Individuals with higher degrees should be recruited more rapidly
- What if:
- \(\tilde{\theta}\), the ‘coefficient of discoverability’
- \(\tilde{\theta}=1\) corresponds to the standard RDS assumption
- \(\tilde{\theta}=0\) corresponds to sampling being independent of degree
The general model
- The size of the population, \(N\), is not known
- For each degree \(k\) there are \(N_k\) individuals in the population with degree \(k\)
- Sampling is done without replacement with \(n_{k,t}\), the number of people with degree \(k\) recruited by time \(t\)
- Between time \(t\) and \(t+\Delta t\) an individual with degree \(k\) is sampled with probability
\[\begin{align} \lambda_{k,t} & = \frac{\beta_k}{N}I_t(N_k - n_{k,t})\Delta t + o(\Delta t)\ \end{align}\]
- \(I_t\) is the number of people already recruited and actively trying to recruit (invite) new individuals, and the constant \(\beta_k\) is a degree dependent recruitment rate
Estimates from real datasets
| Dataset | \(\theta\) |
|---|---|
| Uganda | 0.35 |
| Cornell WebRDS | 0.05 |
| Pakistani immigrants | 0.53 |
| Jazz musicians in NYC | 0.51 |
- Recruitment rates are neither simply proportional to degree, nor independent of degree
Homophily
- RDS can also provide information on homophily
- Project FLOW
- Between August 2005 and January 2007, RDS was used to recruit 449 individuals (25 seeds, 424 recruits)
- Men who have sex with men and/or
- Drug users (used methamphetamine, cocaine and/or heroin) in Los Angeles, California
- Individuals were tested for HIV, and asked whether they had sex with their recruiter
RDS tree
Homophily in Project FLOW
Transmission
- Transmission occurs over the contact network, and so may contain at least some information on the contact network
- For acute infection, the time of onset of infection can help infer transmission
- For recurrent infection, information on first and subsequent infection can be informative
- For rapidly evolving pathogens, sequence data can provide information
- Trees
- Networks in the presence of pathogen recombination
Time between measles infections in households
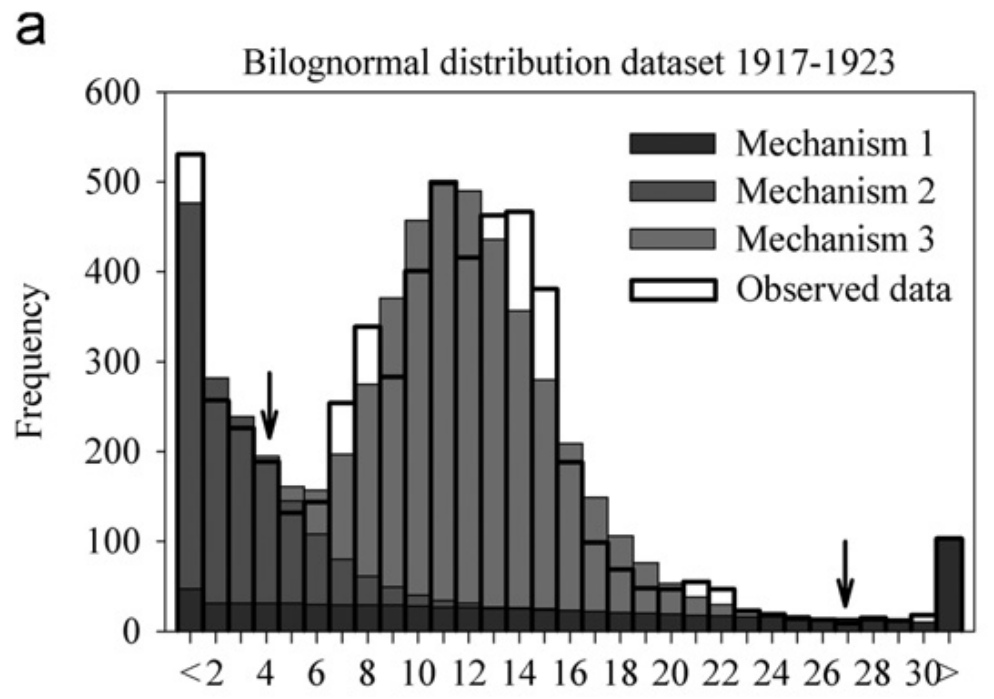
Klinkenberg and Nishiura (2011)
Harnessing sequence data
- Sequence data can tell us about the transmission network, which may inform us about the contact network
- Allows us to ask what could have happened
- Many challenges in harnessing sequence data
- Frost et al. Epidemics (2014)
- Approaches to using sequence data to look at population structure:
- Top down
- Bottom up
- In the top down approach, we compute measures on the tree, and ask whether population structure might have generated the observed patterns
Measures of imbalance
Asymmetry in a HIV sample from a London hospital
Measure of assortativity
A ‘core group’ model of HIV
\[ \begin{align} \frac{dS_1(t)}{dt} & = \Lambda_1 -S_1(t)\left(\beta c_1 p_{11} \frac{I_1(t)}{N_1(t)} + \beta c_1 p_{12} \frac{I_2(t)}{N_2(t)}\right) - \mu S_1(t)\\ \frac{dI_1(t)}{dt} & = S_1(t)\left(\beta c_1 p_{11} \frac{I_1(t)}{N_1(t)} + \beta c_1 p_{12} \frac{I_2(t)}{N_2(t)}\right) - (\mu+\gamma)I_1(t)\\ \frac{dS_2(t)}{dt} & = \Lambda_2 -S_2(t)\left(\beta c_2 p_{21} \frac{I_1(t)}{N_1(t)} + \beta c_2 p_{22} \frac{I_2(t)}{N_2(t)}\right) - \mu S_2(t)\\ \frac{dI_2(t)}{dt} & = S_2(t)\left(\beta c_2 p_{21} \frac{I_1(t)}{N_1(t)} + \beta c_2 p_{22} \frac{I_2(t)}{N_2(t)}\right) - (\mu + \gamma)I_2(t)\\ \end{align} \]
A model of mixing
- We consider a mixture of (a) preferential within-group mixing and (b) proportionate mixing using a parameter \(\rho_i\)
\[ \begin{align} p_{ii} & = \rho_i + \left(1-\rho_i\right)\frac{c_i(1-\rho_i)N_i}{\sum_k c_k (1-\rho_k)N_k}\\ p_{ij} & = \left(1-\rho_i\right)\frac{c_j(1-\rho_j)N_j}{\sum_k c_k (1-\rho_k)N_k} \end{align} \]
Core groups can lead to asymmetric trees
Frost and Volz (2013)
Bottom up vs. top down
- Top down approaches are statistically well developed, however:
- Blunt instrument
- Difficult to integrate individual level data
- Bottom up approaches are better suited in this regard
Networks
- We are often interested in networks of infection
- Sequences do not directly provide information on who infected whom
- Loss of directionality
- In many cases, we have not sampled all the individuals in the transmission network
Loss of direction
Volz and Frost (2013)
‘Invisible’ transmissions
Volz and Frost (2013)
‘Transmission networks’ from phylogenies
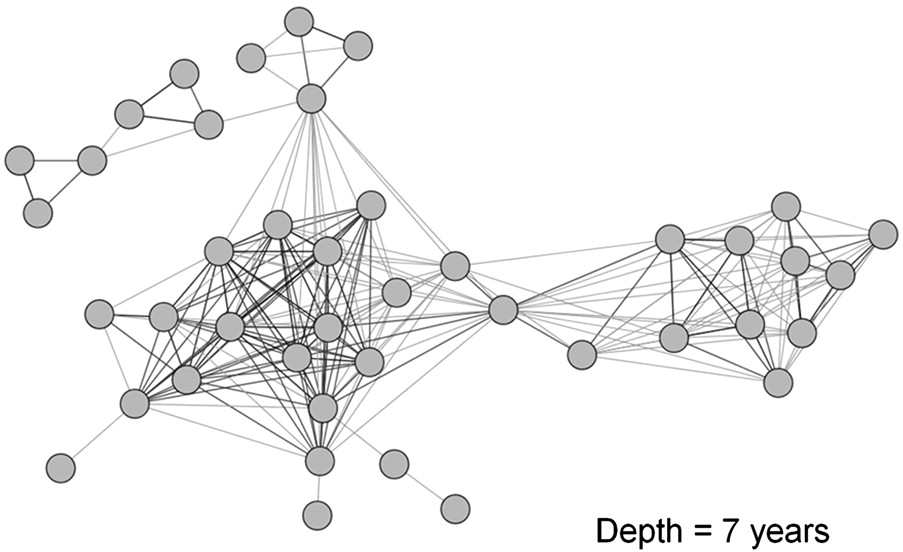
Leigh Brown et al. (2011)
Power law degree distribution
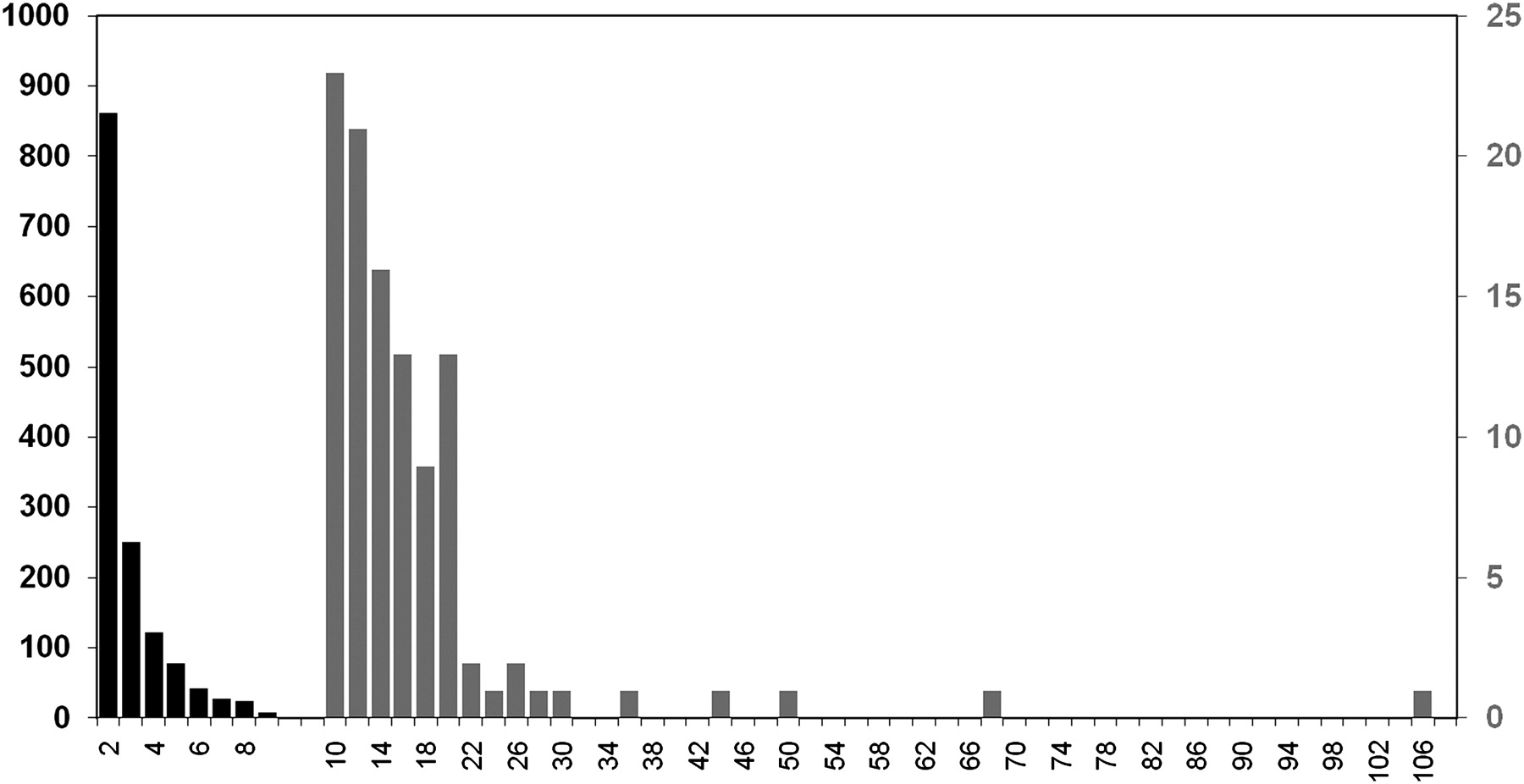
Leigh Brown et al. (2011)
Counting infections
- Let us consider a simple ‘SIR’ type epidemiological model in which we count the number of times an individual has infected another
- No intrinstic differences between individuals
- In the absence of infection, what is the degree of the transmission network?
- Can we infer who has and who has not infected other individuals?
A model
\[ \begin{align} \frac{dS}{dt} & = -\beta S \Sigma_i I_i\cr \frac{dI_1}{dt} & = \beta S \Sigma_i I_i - \gamma I_1 - \beta S I_1\cr \frac{dI_i}{dt} & = \beta S I_{i-1} - \gamma I_i - \beta S I_i\cr \frac{dI_N}{dt} & = \beta S I_{N-1} - \gamma I_N\cr \end{align} \]
Degree distribution is geometric
- Half of individuals at any one time have not (yet) infected anyone
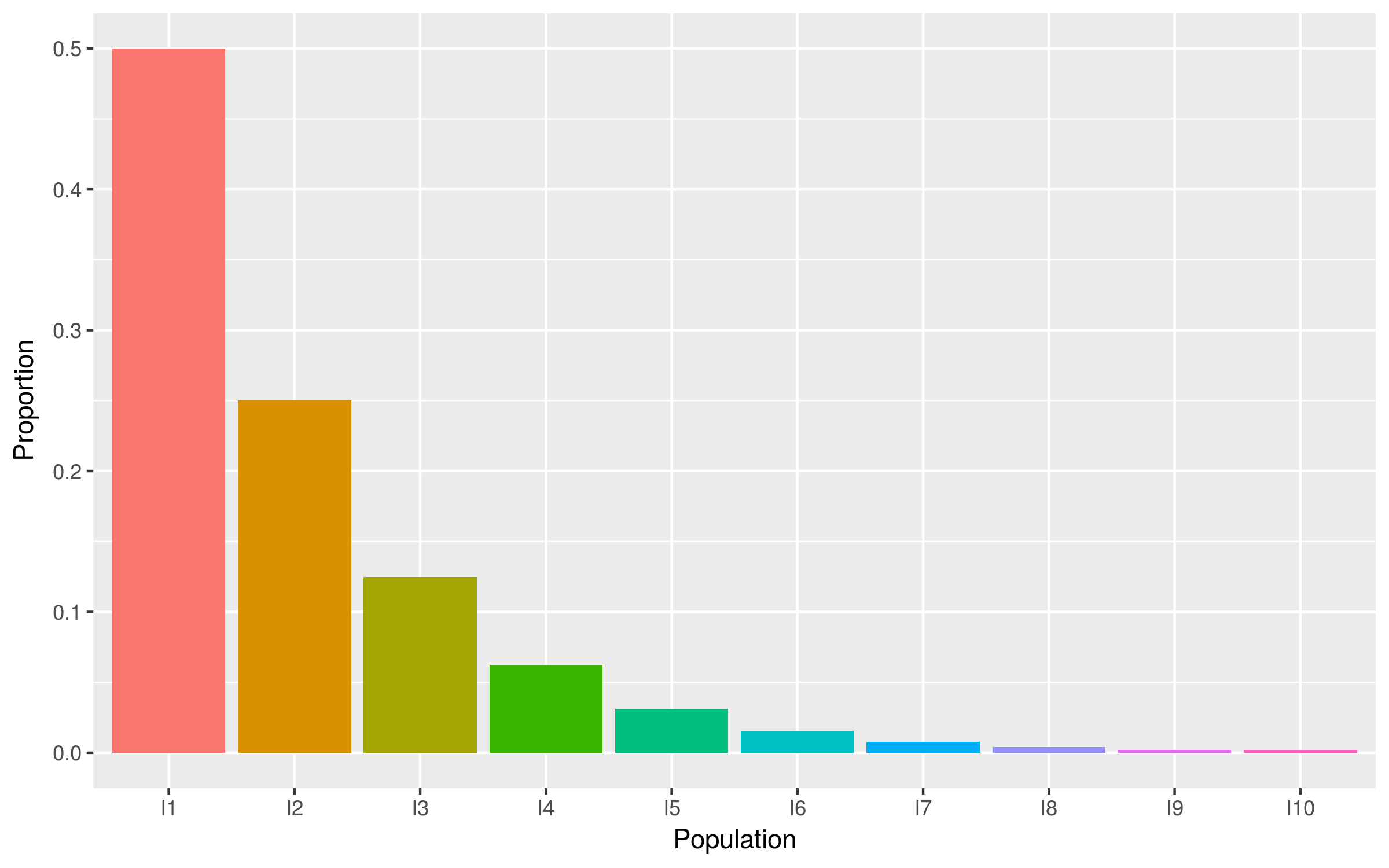
Consequences
- Due to the natural asymmetry in trees, we cannot draw strong conclusions from the degree of a network
- Strongly confounded by time since infection
- We can, however, compare different groups of individuals, allowing us to integrate individual-level data with sequence data
- Better if we can control for time since infection
- Only works with high levels of sampling
Example: red colobus in Uganda


Data
- Individual-level
- Approximate age
- Gender
- Network-level
- Pairwise interactions (aggressive, grooming, etc.)
- Biological
- Host microsatellite data
- Viral sequence data
- Diagnostic
- Phylogenetic
Viruses
- Multiple, co-circulating viral infections
- Retroviruses:
- Simian immunodeficiency virus (SIV)
- Simian T-cell lymphotropic virus (STLV)
- Simian foamy virus (SFV)
- Simian immunodeficiency virus (SIV)
- Simian haemorrhagic fever virus (SHFV1, SHFV2)
- Simian pegivirus (SPgV)
Aggressive interactions and infection
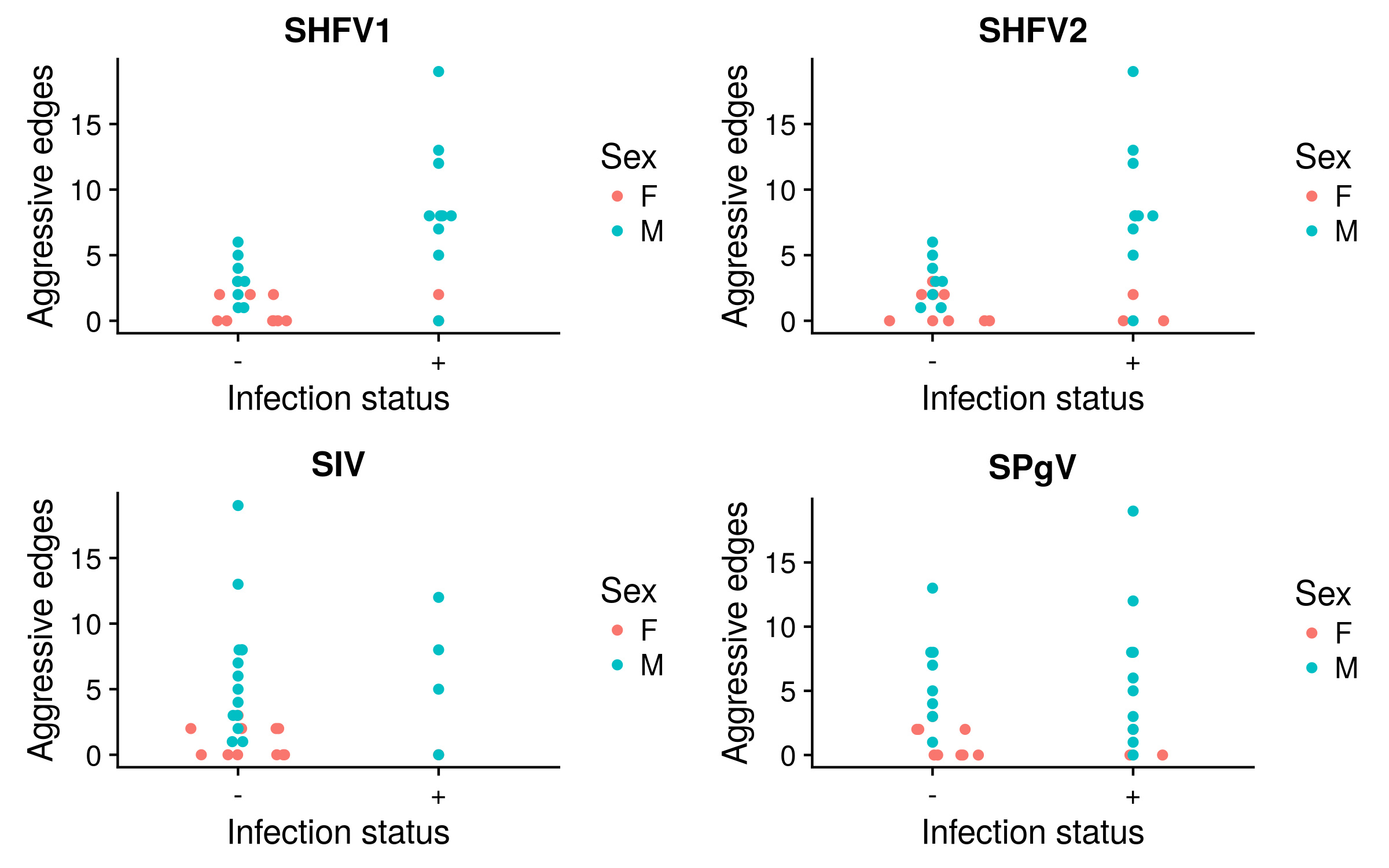
Pairwise relatedness
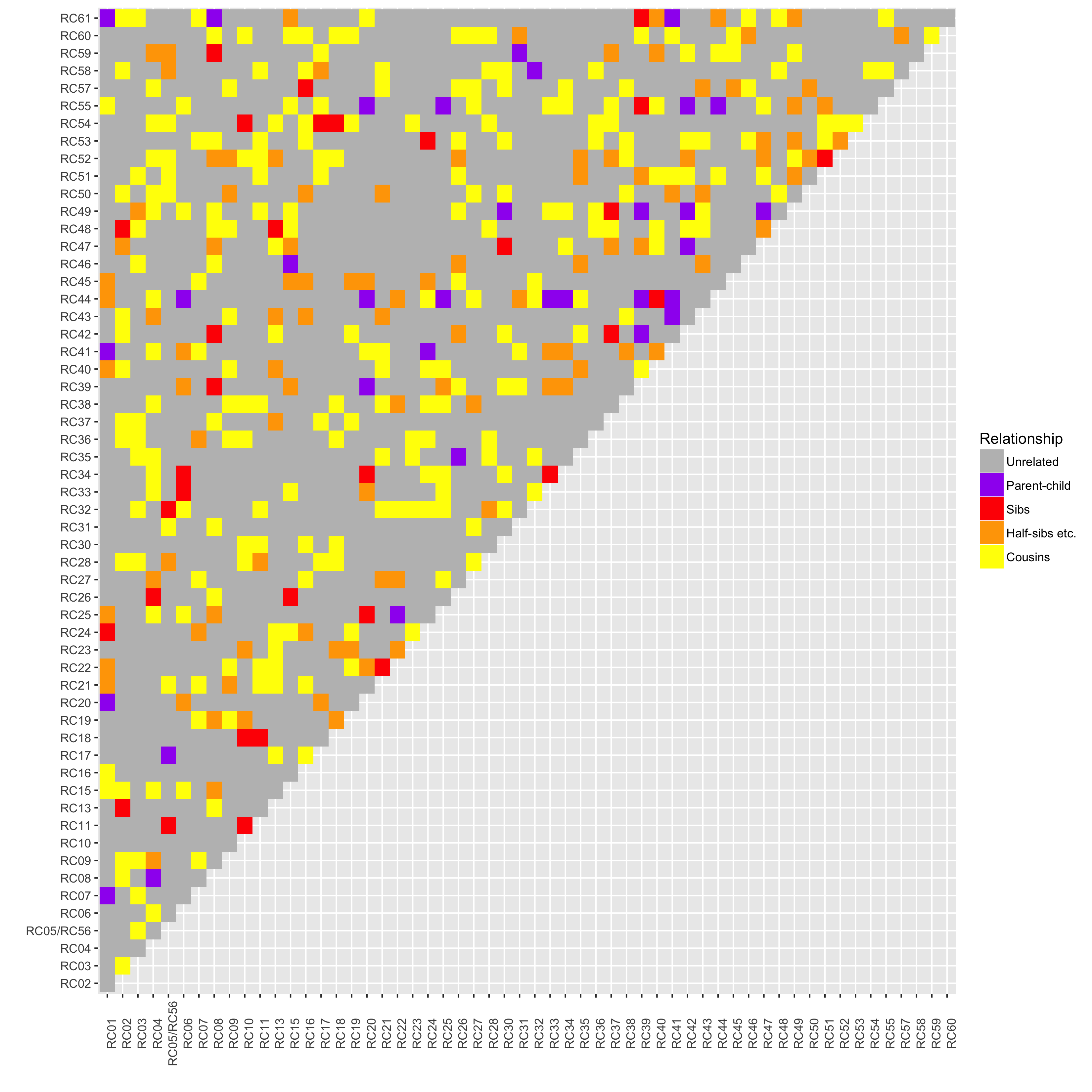
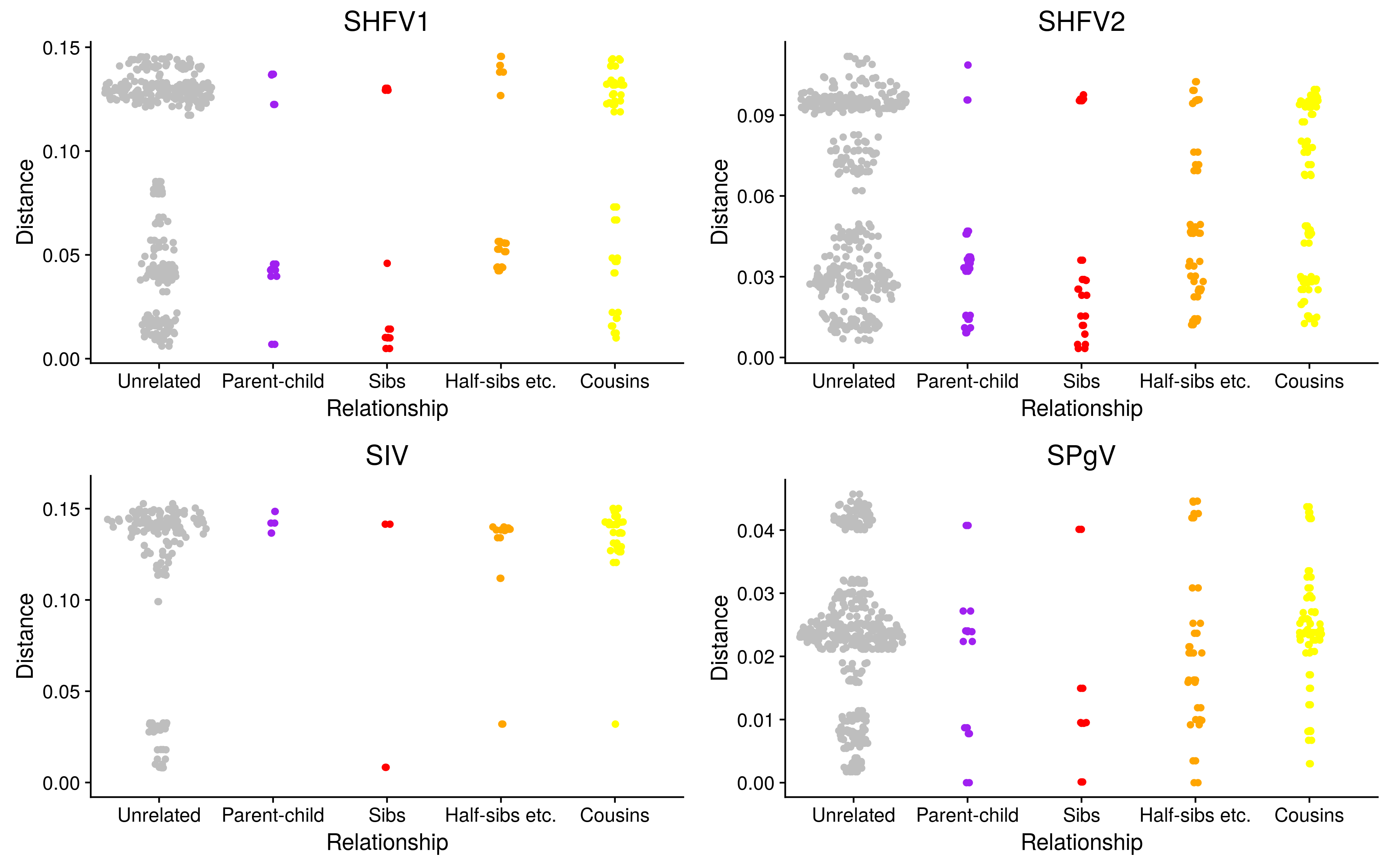
Relatedness and clustering
Co-infection
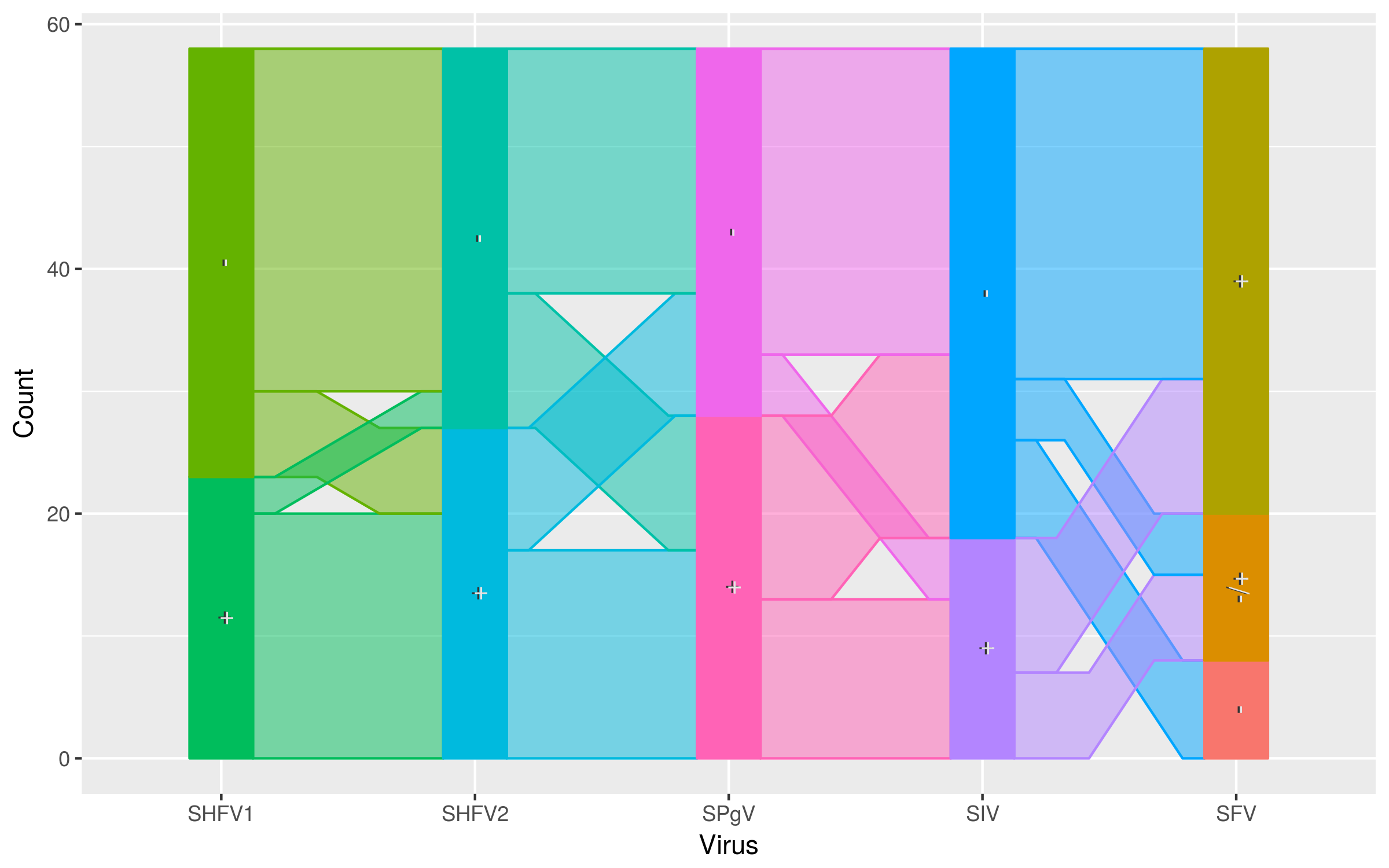
Co-transmission of SHFV
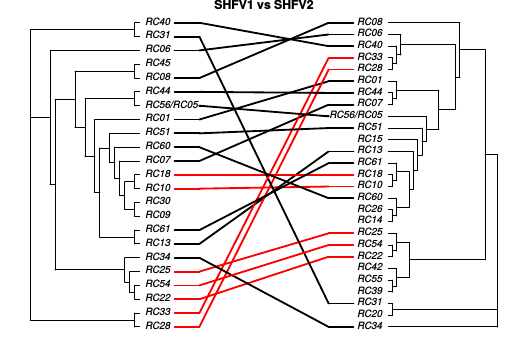
Shared risks with SHFV-1 and SIV
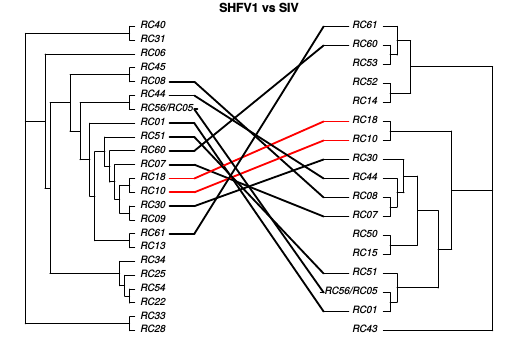
Recombination in SIV
Conclusions
- Networks are important for disease transmission
- It is difficult to obtain accurate information on contact networks
- Esp. for pathogens such as HIV
- Social network data may help to identify hotspots of transmission
- Sequence data provides objective data on transmission
- Best in conjunction with other forms of data
- Lots to do in terms of incorporating multiple infections, recombination etc.
Thanks!



Social network data